
home contact company.overview news sitemap print




Contact |
|
Heiko Steuer |
|

Haemocompatibility of Medical Devices
Medical devices that contact blood during use (e.g. biomaterials, implants) must be haemocompatible. Haemocompatibility can be tested with static or dynamic blood incubation systems depending on the application mode of the medical device.
According to guidance DIN EN ISO 10993-4 tests are grouped into five test categories:
- Coagulation
- Platelets
- Haematology
- Complement
- Thrombogenicity
The NMI TT consults with clients about tests needed and offers tests in all test categories and with both incubation systems.
Chandler Loop Method
The Chandler Loop method simulates the circulation of blood by rotating tubes (see picture on the right). Therefore the Chandler Loop incubation is the method of choice to test haemocompatibility for medical devices placed into the blood stream.
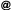
 +49 7121 51530-839
+49 7121 51530-839